It has been a little while since I posted on the blog, so I figured I would get some new pertinent chemistry and talk about it. When I saw this article, I knew I had to talk about it because it was a paper that had elements in green chemistry, renewable feedstocks and pharmaceuticals. That never happens. Or maybe I haven’t found too many papers that cater to all these areas. So the most important aspect of this paper is where the starting material came from and what it was made into. The paper was found in Green Chemistry, “Synthesis of ranitidine (Zantac) from cellulose-derived 5-(chloromethyl)furfural” by Mark Mescal et al, Green Chemistry, 2011,13, 3101-3102, DOI: 10.1039/c1gc15537g. Once again, I am beating the press before they print so I supplied the Digital Object Identifier. I am sure the sales for Ranitidine are quite large; who doesn’t get heartburn at one time or another. I think it is very fortunate the author shows you can use a starting material that can be derived from just about any source of cellulose. I find it interesting how renewable feedstocks can be utilized in industry and become part of important commodities, such as plastics, pharmaceuticals, etc. This paper refers to another discussing where the starting material was derived from. Starting material can be sugars, cellulose or raw cellulosic biomass and the reaction can produce yields of 80-90 %. M.Mascal and E. B. Nikitin, Angew. Chem., Int. Ed., 2008, 47, 7924;  On with the show, though. The original synthetic route was provided in the paper and I will provide it to you.
On with the show, though. The original synthetic route was provided in the paper and I will provide it to you.
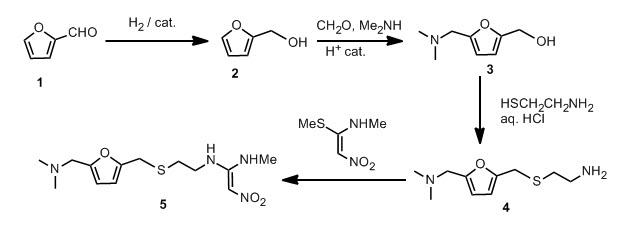
Furfural 1 was reduced to give the furfuryl alcohol 2. The furfuryl alcohol is methylaminated to give 3, which is reacted with cysteamine in concentrated HCl to give 4. This is condensed with 1-methylthio-1-methylamino-2-nitroethylene to give the final product. The patent literature has the yield < 50 % for the aminomethylation and subsequent reaction with cysteamine, but recently, these steps have been reported to have higher conversions.

This new synthesis, apart from using a renewable feedstock as a starting material, has synthetic steps with an average yield of 91 %, and requires no chromatography. Note that N-acetylcysteamine was used as opposed to cysteamine in the first step, in high yield. A reductive amination with methylamine gives 8 again in high yield. Treatment with KOH provides the free amine 9 and the final step is the condensation with the nitroethylene used in the previous synthesis. I thought it was a very nice synthesis.
This was originally posted on PHARMNBIOFUEL.COM on 2011-10-20. I will be coming out with a new posting really soon. Hold on. You can subscribe to this blog using an e-mail alert, follow DevelopProcess on Twitter, look for my posting on Google+.