Hi Everyone. I was trying to think of a theme for this newest post and realized that I had recently seen Queens of the Stone Age and they had a song called “Go with the Flow” and that is what I am going to talk about today, flow chemistry. I have always been fascinated with continuous flow and how it can be used to reduce several issues that you have with batch chemistry work. One of the advantages that a flow reactor has over a batch reactor is the ability to suppress exothermicity of reactions. There is better mixing times and low residence time of reactants. It should be of no surprise that the following paper “Continuous Flow Magnesiation of Functionalized Heterocycles and Acrylates with TMPMgCl·LiCl” by Trine P. Petersen, Matthias R. Becker, and Paul Knochel*, that appears in Angew. Chem. Int. Ed. 2014, 53, 7933 –7937, doi: 10.1002/anie.201404221 deals with this topic. I have only developed batch processes, so I can only look at this work with fascination. I recall that lithium chloride could be used in Grignards to form TurboGrignards through Knochel’s work, so it was with some curiosity that I wanted to look at this work.
The paper talks about using the THF-soluble base, TMPMgCl·LiCl with a variety of heterocycles (and acrylates). One of the points, I found fascinating was the metalation of this chlorothiophene. I can’t remember specifics, but from my past, I remember one of our team members was making a similiar molecule using sec-BuLi and needing cryogenic conditions to lithiate and then trap the lithiated thiophene with carbon dioxide to get the thiophene carboxylic acid. Maybe this could have helped us back then.
The setup used in the paper involves three pumps, one for the substrate, one for the TMPMgCl and one for the electrophile. Reactions were performed in either a glass-chip reactor or a coil reactor. The scale of the reaction is 1.7 mmol, but this is scalable to 45 mmol without any further optimization. 1.1-1.2 equivalents of TMPMgCl·LiCl are used in each experiment.

This is a glass chip reactor (I was curious what they looked like). It has a 1 mL volume and 3 ports.
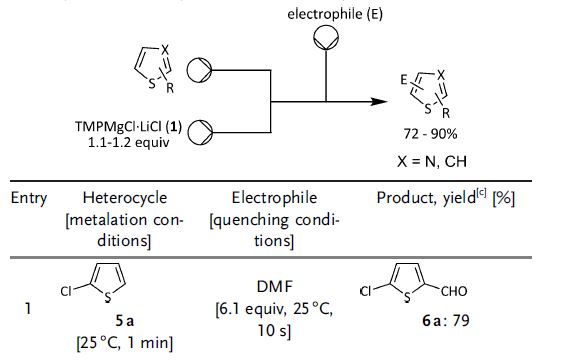

I think it is quite remarkable that metalation of heterocycles can be performed at reasonable temperatures, taking into account that you would need specialized flow equipment to carry these reactions out. Comments are always appreciated.
You can follow me on many different platforms, Twitter (@DevelopProcess), Google+(Brian Halden), by e-mail (sign up on the site).
Have a great weekend.
Unfortunately, much of Professor Knochel’s chemistry is patented, eg., the RMgX-LiCl composition of matter in US7384580. This little tidbit rarely comes out at conferences or in papers. Knochel had apparently licensed this art to the old Chemetall, which was bought out by Rockwood Lithium. Recently, Rockwood Lithium merged with Albemarle who seems to be seeking world domination of the lithium market. Who knows what the availability will be once the merger settles down. This does little to quell my concern about professors patenting art that is subsequently assigned to a university. Do we fund universities to produce art that is then subject to a sanctioned monopoly, that is, a patent? Seems like a funny way to use public funds.
LikeLiked by 1 person
Thanks for the comment. I didn’t realize that bit about Knochel’s chemistry being patented. Was amazed at performing metallations at reasonable
temperatures and not having to worry about the ice bath or chiller that can’t reach -78 C.
LikeLike